Canada approves new vaccine for children under 5
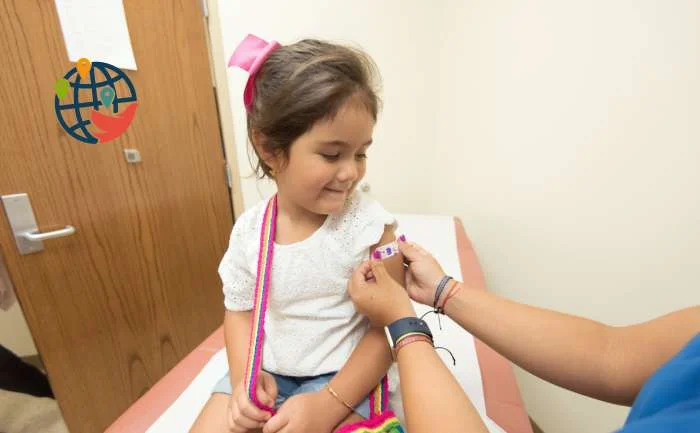
Now 3 doses of Pfizer can be given to children 6 months and older.
On Friday, Health Canada approved Pfizer's pediatric COVID-19 vaccine for children aged six months to four years.
In approving the Pfizer-BioNTech Comirnaty vaccine for children under the age of five, the ministry stated that the benefits of the drug outweigh the potential risks for this age group.
The vaccination schedule is standard: 2 vaccinations 3 weeks apart and a booster after 8 weeks.
The decision was made after reviewing data from a controlled phase 2/3 study of 4,526 children aged six months to four years.
Last month, Canada approved the first booster vaccine against COVID-19 for children ages 5 to 11. Now experts are expecting a new wave of COVID-19 as children return to face-to-face classes and people gather indoors.
Pfizer announced that the company will begin shipping pediatric doses nationwide in accordance with Canadian government and health authority guidelines, but did not specify a timeline.

















